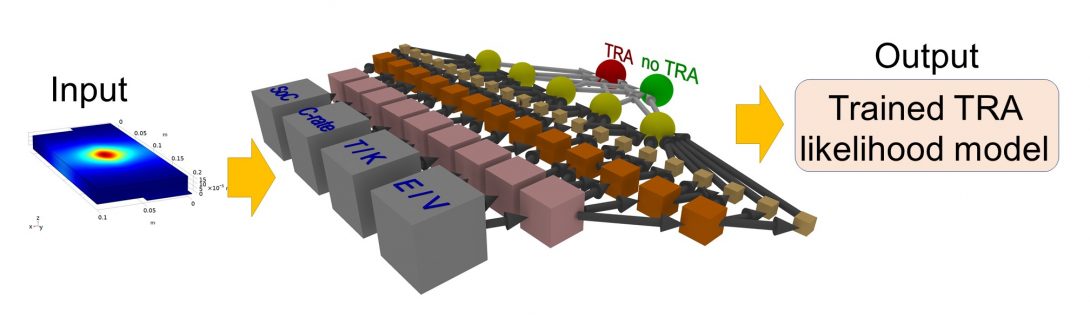
Over the last two decades, the tremendous effort of researches brought the Li-ion battery (LIB), based upon the intercalated graphite electrode, to its advanced level enabling their wide-spread application. Unfortunately, high demand led to many safety problems especially associated with thermal runaway. A very common reason of LIBs’ thermal runaway is the exothermic decomposition of solid electrolyte interface (SEI) leading to a battery’s rapid self-heating and in extreme scenarios to explosion. It is very challenging to suppress, control or sometimes even observe the thermal runaway due to the SEI formation/decomposition in realistic operation conditions. However, the evolution of thermal and electric parameters during LIBs operation may indicate the occurrence of thermal runaway, which will allow its early forecasting and corresponding suppression.
In order to predict the thermal runaway in LIBs, we use machine learning (ML) techniques, in particular, convolutional neural network (CNN) and deep neural network (DNN) to identify temperature pattern change leading to the thermal runaway. The ML methods are trained based upon the images obtained from the multi-physics calculations using Comsol software package. The pouch type LIB consisting of five distinct layers, i.e., two current collectors, negative and positive electrodes and electrolyte, is considered. The electrochemical model is based on a 1D continuum description of reaction and transport along electrodes, electrolyte and current collectors, plus an additional dimension in the electrode particle (P2D). The SEI formation/decomposition is modeled using the parasitic current approach. We investigate three regimes of thermal runaway occurrence, i.e., a single heat source, two heat sources and multiple sources. Corresponding, different CNN and DNN architectures were built and trained based upon the images from multi-physics calculations. The prediction of the trained network has been tested using simulated and literature available data.
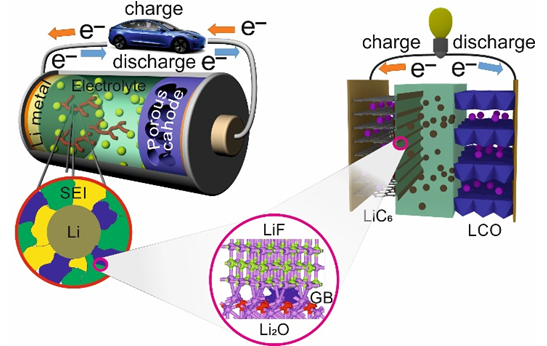
Using a phase-field modeling (PFM) study of lithium (Li) electrodeposition process in Li‒metal battery (LMB) to explain its physics and morphology. Three regimes are investigated: Li filaments evolution, Li bush-like structure evolution and the transition between Li filaments and bush-like morphologies. The model takes into consideration the effect of solid electrolyte interface (SEI) on Li electrodeposits evolution. Also, an important new element of the model is the capability to represent the directional diffusion of Li by implementing diffusion tensor of Li-ions in the electrolyte, thus, allowing to simulate Li filaments root growth. In addition, an elastic deformation energy of the Li solid phase is included in the free energy functional of the PFM, which allows for monitoring the stress field and its influence on Li filaments/bush-like structure evolution. In particular, a significant stress is observed at the root of the Li electrodeposits, which can support the development of the experimental strategies to suppress their formation by lowering the stress field. Thus, the present study, in addition to improving the fidelity of the PFM of Li electrodeposition, identifies critical regimes of Li filaments growth and splitting, allowing for a more profound understanding of their influence on battery performance.
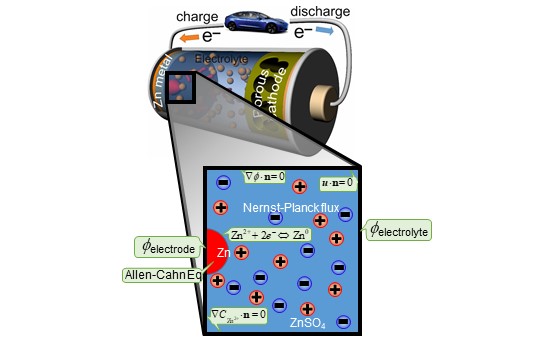
Zinc (Zn) aqueous rechargeable batteries (ZB) have showed a tremendous success in various applications, however, analogous to their Li-ion battery counterparts, they suffer from the dendrite formation and eventual decrease in capacity. In our current work, we present the results from phase-field modeling (PFM) of Zn electrodeposition at different current densities. Different Zn morphologies, such as boulders, whiskers and dendritic shape are modeled. The computational model predicts two-dimensional distribution of Zn electrodeposits, Zn2+ ions concentration, electrostatic potential, stress and equivalent plastic strain. It was found that the stress has a major influence on the electrodeposition, and vice versa the Zn electrodeposits growth is found to affect the stress distribution significantly. The plastic yielding occurs preferentially at the node of the boulders and through the filaments and dendritic structures. The results also provide a basis for development and design of novel strategies against unwanted Zn dendrites formation.
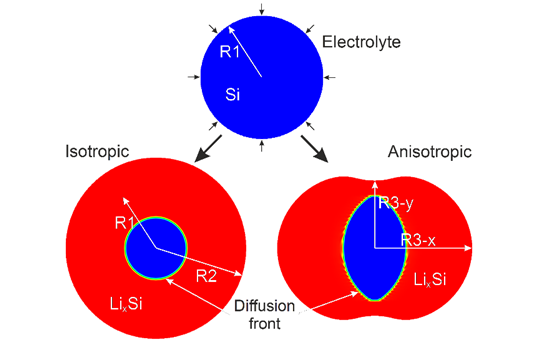
Modeling Elasto-Plastic Deformation in Lithium-ion Batteries: Si electrode
The understanding of elasto-plastic deformation is crucial for performance and durability of Lithium (Li) ion batteries. In our group, we are developing a generic framework to describe kinetics of alternative electrodes (e.g. Silicon) lithiation and its effect on structural changes, stress evolution and mechanical properties. Two models have been developed, where the first approach is a coupled finite element elasto-plastic model using ANSYS finite element package, and the second is a phase-field model based upon MOOSE framework. The models include the description of the lithiation induced phase transformation, morphological evolution and stress generation in Si nanowires.
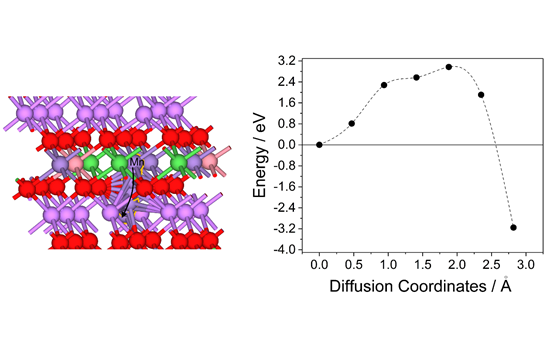
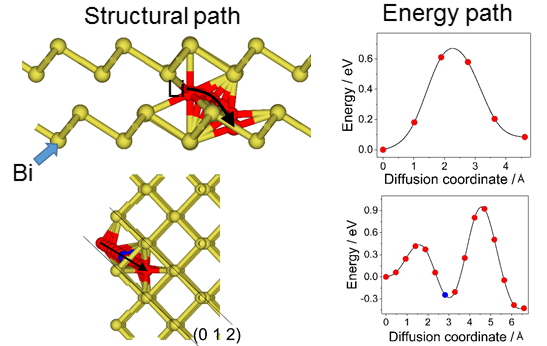
DFT calculations of (de)alloying in Lithium-ion Batteries: Bi electrode
Materials storing energy via alloying reaction are promising anode candidate in rechargeable lithium-ion batteries (LIBs) due to their much higher energy density than current graphite anode. Among these materials, bismuth (Bi) provides a high theoretical volumetric capacity of 3800 mAh·cm−3 when it alloys with Li+ forming LixBi composites. Using the DFT method, we studied the favorable pathways for Li diffusion into the bulk of Bi as well as the intercalation path from the most stable surface into the bulk of Bi.
Despite the high energy density, challenges such as rapid capacity degradation and voltage decay have limited the widespread application of Li-rich layered cathode (LLC) materials. Thus, it is critical to fully understand the degradation mechanisms that contribute to the lower structural stability of Li-rich cathodes compared to the conventional layered oxide cathodes. We use density functional theory (DFT) calculations to investigate atomic arrangement of metals in the NMC cathode to reveal degradation mechanism of these materials.